- Common Isotopes Of Hydrogen
- 3 Isotopes Of Hydrogen Similar
- Atomic Mass Of Hydrogen Isotopes
- Write 3 Isotopes Of Hydrogen
- Uses Of Isotopes
Isotopes of hydrogen
Common Isotopes Of Hydrogen
Hydrogen is the first element in the periodic table. It has the simplest electronic configuration 1s1. It contains one proton in the nucleus and one electron.
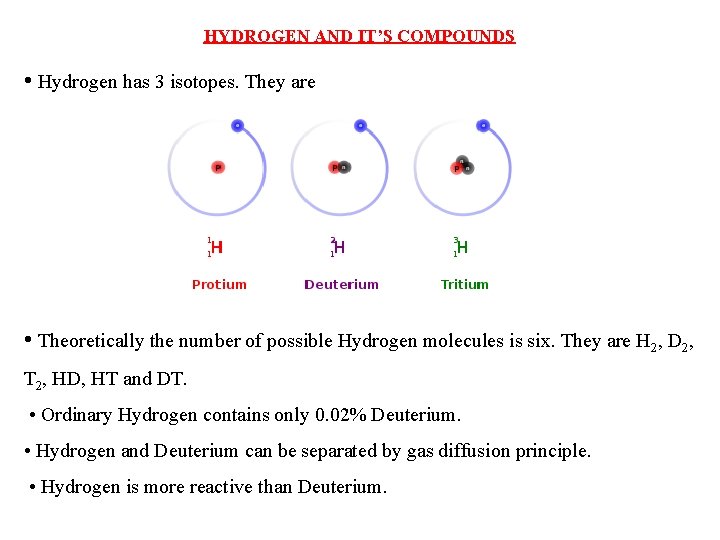
- Hydrogen has the atomic number one and is the first element in the periodic table. The isotopes are those elements that have the same atomic number but a different mass number and there are three hydrogen isotopes such as protium 1H1, deuterium 1H2, or D and tritium 1H3.
- Hydrogen has three naturally occurring isotopes, denoted 1 H, 2 H and 3 H. Other, highly unstable nuclei (4 H to 7 H) have been synthesized in the laboratory but are not observed in nature. 1 H is the most common hydrogen isotope with an abundance of more than 99.98%.
- Parameter Gaseous Normal Hydrogen Gaseous para-Hydrogen; density at 0 °C, (mol/cu cm)X10+3: 0.04460: 0.05459: compressibility factor at 0 °C: 1.00042: 1.0005.
- Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. Mass numbers of typical isotopes of Hydrogen are 1; 2. Common Isotopes of Hydrogen. The most abundant isotope, hydrogen-1, protium, or light hydrogen, contains no neutrons and is simply a proton and an electron.
Isotopes:- Atoms of the same element having same atomic number but different mass number are called isotopes.
Further data for naturally occuring isotopes of hydrogen are listed above. This table gives information about some radiosotopes of hydrogen, their masses, their half-lives, their modes of decay, their nuclear spins, and their nuclear magnetic moments. Isotope Mass / Da Half-life Mode of decay Nuclear spin Nuclear magnetic moment; 3 H: 3.
There are three isotopes for hydrogen with mass numbers 1, 2 and 3, each possessing an atomic number of one.
The structure of the three isotopes of hydrogen are
3 Isotopes Of Hydrogen Similar

1.Protium or ordinary hydrogen: It is the common form of hydrogen. Itconsists of one proton in its nucleus and one electron revolving around it. It constitutes 99.984% of total hydrogen available in nature. Its mass number is one.
2.Deuterium or heavy hydrogen: 1H2or1D2. It occurs naturally in verysmall traces. The proportion present in naturally occurring hydrogen is in the approximate ratio: D: H~ 1:6000. It's nucleus consists of a proton and a neutron. However only a solitary electron is revolving around the nucleus. Its chemical properties are similar to those of protium but their reaction rates are different.
Atomic Mass Of Hydrogen Isotopes
3.Tritium, 1H3 or 1T3: It occurs in the upper atmosphere only whereit is continuously formed by nuclear reactions induced by cosmic rays. Unlike deuterium, it is radioactive, with a half-life of ~ 12.3 years. It's nucleus consists of one proton and two neutrons.
They will have same similar chemical properties, however, their reaction rates will be different and their physical properties differ appreciably.
Isotopes of hydrogen :
S. Atomic Mass Number of Percentage
Write 3 Isotopes Of Hydrogen
Name Symbol
No number number Pro- Neu- abundance
tons trons
1. Protium or 1H1 1 1 1 0 99.984

hydrogen
2. Deuterium 1H2 1 2 1 1 0.016

or heavy
hydrogen
3. Tritium 1H3 1 3 1 2 10-15
Uses Of Isotopes
